Point-of-Care Testing and its emerging trends
What is Point-of-Care Testing?
Point-of-care testing (PoC testing) is known to be the medical testing performed by professionally trained healthcare (non-laboratory) workers at or close to the location of patient treatment (NHS). These examinations frequently include blood and urine tests. At the patient’s site or close by, PoC testing aims to collect samples and produce reliable results in a brief amount of time. As a result, PoC testing can be used in a variety of primary care, community, and secondary care settings, allowing for convenience, quicker results, higher access and possibly lower costs.
Various point-of-care testing devices are available, ranging from simple urine dipsticks and portable glucose meters to complex benchtop analysers for blood gas measurement in critically ill patients. Common point-of-care tests include blood glucose monitoring and at-home pregnancy tests, while tests for haemoglobin, haemoglobin A1c, and PT/INR are frequent for warfarin users. (Lab Tests Online UK).
Point-of-care testing has the potential to disrupt traditional methods, enabling early diagnostics, reducing hospital visits, alleviating laboratory and physician workload, allowing first responders to collect patient data pre-hospital, and offering potential financial benefits.
For example, Point-of-Care testing proves great benefits in the diagnosis for patients with Ebola, as early isolation and care of patients with suspected symptoms are keys in preventing this deadly disease, in highly remote areas. Using the traditional laboratory methods, including collecting samples, transporting samples to laboratory with PCR-based tests, result communication back to sites, could take more than 6 days for the results to be received, which then could be too late for the patients. However, the WHO-approved ReEBOV Antigen Rapid Test Kit could detect Ebola protein, instead of nucleic acid, in less than 15 minutes, correctly identifying about 92% of Ebola infected patients and 85% of those not infected with the virus.

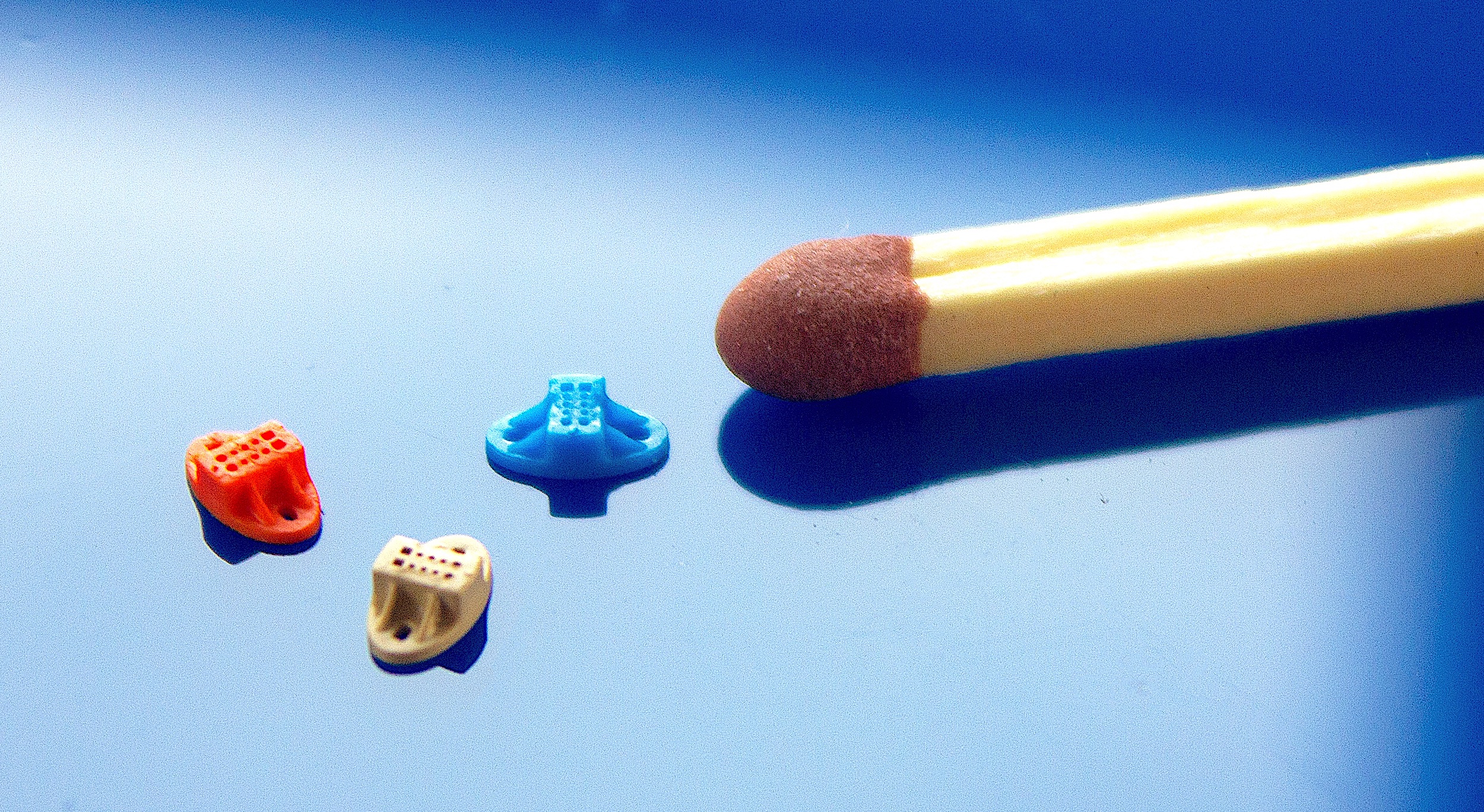
Microfluidic chip with integrated optics features (Photo: Micro Systems)
How big is the market for Point-of-Care Testing?
The necessity for decentralised healthcare services, growing demand for fast testing, and technological improvements have all contributed to the industry’s tremendous rise during the past decades. The Global Point of Care (POC) Diagnostics/Testing Market was estimated to be worth $43.2 billion in 2022, and a recent report projects the market to grow to $98.13 billion by 2030. The market is expected to expand at a CAGR of 10.8% from 2023 to 2030.
The increasing prevalence of chronic illnesses and the COVID-19 pandemic have significantly driven the demand for point-of-care (PoC) diagnostics. Rapid testing is essential for swift identification and infection control. Additionally, the integration of digital health enhances PoC testing, improving data management, patient outcomes, and healthcare experiences through continuous communication between patients, healthcare professionals, and labs, fostering better care coordination and data-driven decisions.
Innovations and Movements in Point-of-Care Testing
Technology is revolutionising the diagnostics sector, with exciting innovations taking place globally.
Artificial Intelligence (AI) is being widely integrated in Point-of-Care Diagnostics for testing. By integrating machine learning (ML) onto Point-of-Care testing devices (Edge interference), data can be collected and processed locally rather than on the cloud, at the point of care. Due to the accessibility of specialised hardware, edge inference is now practical and allows for real-time processing, greater data security, and a decreased dependency on network quality. For instance, the technology is used by a portable retinal camera created in Taiwan to identify diabetic eye illness, enabling primary carers to make diagnoses that would traditionally be made by an ophthalmologist, with the same accuracy level but 10 times faster than a rival cloud-based product and do not necessitate transportation to an expert ophthalmologist. Another example is the use of AI in point-of-care cardiac ultrasound exams, such that medical workers who lack sonography knowledge may nevertheless acquire cardiac ultrasound pictures at Point-of-Care testing with the help of the UltraSight real-time AI guiding programme. According to UltraSight, the technology will give hospital personnel the ability to progress patient triage and treatment with more effectiveness and clinical assurance. By introducing cardiac ultrasonography into local communities, it can also improve access to care for people with chronic heart disease, potentially enhancing patient adherence to crucial therapies.
The Covid-19 has proven to us the importance of remote healthcare, where patients can receive services without getting access to healthcare facilities. The integration of telehealth in Point-of-Care testing helps advance remote patient diagnosis and monitoring, removing the need for travelling and reducing the long waiting time for appointments. For example, the miniaturised diagnostic platform developed by PixCell Medical for CBC test helps patients receive the results within 5 minutes, using one drop of blood, without the need for needle and minimised anxiety for children and parents.
There are also innovative breakthroughs in the field of Point-of-Care testing with the use of microfluidics and lab-on-a-chips. For example, a potential tool for assessing nutritional status at the point of care is the Cornell NutriPhone, as it takes around 15 minutes to determine the levels of iron, vitamin A, vitamin D, vitamin B12, and vitamin C from a single drop of blood. Major OEMs have also developed PoC devices that can run multiple tests on one cartridge, in less than an hour, in the doctor’s office, giving huge benefits to patients as they could have discussions with doctors about their situations in such a shorter time.
Moving forward, education is a key factor in enabling the applications of Point-of-Care testing. Online materials like the knowledge portal, www.myPOCacademy.com, with content developed in collaboration with an outside expert faculty, offers a variety of multimedia learning alternatives and professionally approved training. The website’s clinical specialties covered include cardiovascular disease, diabetes, and respiratory health.

Microfluidics features (Photo: Micro Systems)
Challenges of Point-of-Care Testing
Point-of-care (PoC) testing is likely to grow in importance as individuals take more responsibility for their healthcare, particularly those with limited access to facilities. However, challenges include differences in methodology between PoC and laboratory systems, reliability issues (due to user errors), and the management of supplies and reagents. To ensure optimal treatment, PoC tests should be integrated within a broader testing continuum that includes centralised clinical labs and healthcare professionals.
The management challenges of PoC testing include connectivity between devices and laboratory information systems, as well as operator training. Integrating appropriate software into PoC devices enables real-time result updates to health records, maximising the benefits of quick results and reducing manual entry errors. Operators must undergo thorough training and certification to minimise human error and ensure effective support for local communities and health centres. Establishing a network of PoC systems in regional areas could enhance support, improve cost-effectiveness, and optimise connectivity between centres.
A key challenge for PoC testing is device design, which must prioritise end-users (patients, physicians, etc.) by integrating usability engineering and human factors into the design process. This ensures accuracy, efficacy, user satisfaction, and safety. Techniques such as design research and cognitive science allow experience designers to address both physical and digital interactions, creating efficient, user-focused designs from the outset. Adopting a human-centred design approach can also reduce development time and costs by minimising later-stage iterations.
The recent changes in lifestyles after the Covid-19 has proved the rising importance for the use of Point-of-Care tests, especially for those who have limited mobility, for in areas with limited access to complex laboratories. There are still a number of challenges for the development of remote diagnostics, mainly in R&D and infrastructure, it will not be long until Point-of-Care testing devices are available in patients’ homes, or in local clinics in the most remote areas.

Micro Systems’s vast know-how in design, ultra-precision micro machining capabilities and expert knowledge in micro moulding technology allow us to manufacture advanced microfluidic moulds with tolerance as low as +/-0.001mm, with integrated optics. We have a dedicated micro moulding facility, and have ISO13485 and ISO9001 certifications. For more information, please Contact us or visit our website.