Sustainability in medical device design and production
Medical device is vital to human health, improving public health and addressing emergencies. As we strive for sustainability, it’s essential for suppliers to innovate and adopt responsible practices in design and production of medical device.
The environmental problem of medical device
According to WHO, a medical device is defined as “any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use, software, material or other similar or related article, intended by the manufacturer to be used, alone or in combination for a medical purpose”. In the UK, 66% of the NHS’s total carbon emissions is due to its supply chain, including pharmaceuticals, medical and non-medical devices.
Due to stringent industry regulations, it is often more cost-effective to discard medical devices than undergo the complex and expensive process of cleaning, sterilizing, and disinfecting. While manufacturers focus on environmental sustainability, they must balance this with ensuring product safety and efficacy, often resulting in the use of single-use plastics. As a result, a significant portion of medical device waste comprises single-use components.
The entire lifecycle of medical devices—from production to disposal—has substantial environmental impacts. Carbon footprints increase due to raw material procurement, production, transportation, sterilisation, and disposal, especially with the incineration of single-use plastics. The growing complexity of devices, including electronic components and diverse materials, further complicates recycling and disposal, exacerbating global waste challenges.
In 2017, out of 5.3 million tonnes of hazardous waste collected in England, 35% was recovered, 21% was transferred, 23% was treated, 6% was incinerated and 14% was sent to landfill, especially, the amount sent to landfills were significantly lower compared to the data in 2000, proving potential opportunities to tackle the problem of medical device waste.
Sustainability in medical device
While often associated solely with environmental protection, sustainability also encompasses social and economic dimensions. The Brundtland Commission (1987) defines sustainability as “meeting the needs of the present without compromising the ability of future generations to meet their own needs.” By integrating sustainable practices into medical device design and manufacturing, companies can reduce environmental impact, enhance customer appeal, achieve cost savings, attract investors, and strengthen brand and competitive positioning.
Sustainability in medical devices begins at the design phase, where around 80% of environmental impacts are determined. Early design decisions significantly influence a product’s sustainability throughout its lifecycle. Key factors include reducing carbon emissions, energy consumption, water use, and material waste across the entire lifecycle, from design and material selection to manufacturing, supply chain management, and distribution.
When selecting materials for medical devices, designers and engineers must consider factors such as renewability, resource efficiency, emissions, ethical sourcing of raw materials, transportation impacts, degradability, and reusability. Recently, biopolymers like polyhydroxyalkanoates (PHA), which are biocompatible and biodegradable in the body, and polylactide (PLA), derived from corn starch and more biodegradable than petroleum-based plastics, have emerged as popular alternatives to traditional plastics. By collaborating with tool manufacturers and suppliers, clients can choose materials that enhance the sustainability of their products.

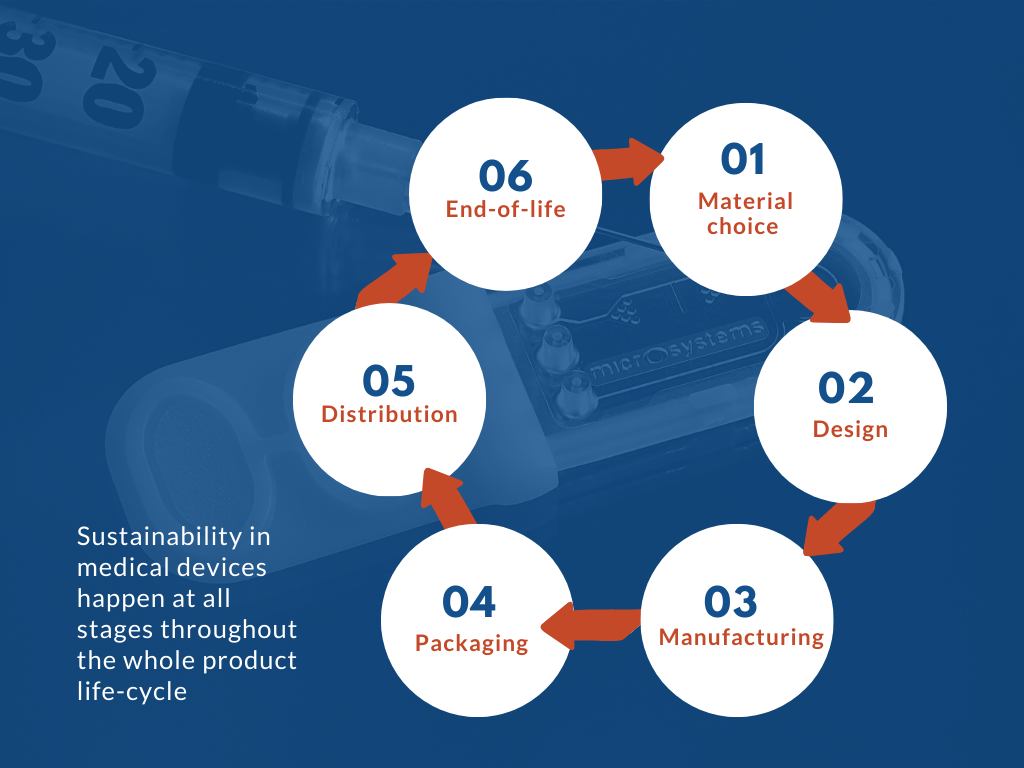
In general, medical device designers, manufacturers and users should constantly challenge themselves throughout all stages of the products’ life cycles:
- Material choice : The material choice for medical devices significantly impacts their entire lifecycle. While plastic offers benefits like lightweight for easier transport and durability for long lifecycles, it can take 20 to 500 years to degrade, depending on the material and environmental conditions. Selecting materials should prioritize suitability, environmental impact, recycled content, responsible sourcing, and end-of-life treatment.
- Can a petroleum-based plastic be replaced with a bio-based material?
- Should the number of different materials utilised be reduced to make the device easier to be recycled at the end of its lifecycle?
- Should the plastic part be replaced with glass or stainless steel so that it can be repurposed and reused?
- Where does the material come from? How transparent is the material? Is the material available locally? Who are involved in the production of the material?
- Design : Medical devices are becoming smaller and more advanced to meet market demands while reducing costs and resource use. By adopting sustainable design, companies can minimise environmental impact, optimise efficiency, and support socially responsible practices throughout the product lifecycle.
- Can I decrease the overall size or weight of the device to reduce material consumption, packaging, and carbon emissions associated with distribution?
- Can the design be improved to increase the lifespan of the device? How durable is the design?
- How to make the device easy to maintain?
- Can electronic components be shrunk to lessen their final disposal impact?
- Is it possible to lower the battery size or convert to a more sustainable battery technology (for example, rechargeable batteries)?
- How to reduce the amount of different materials used?
- Manufacturing : It is crucial for manufacturers to measure, analyse and improve their technologies and processes, such that they can control their impacts on the environment, while increasing productivity and reducing time to market, overall helping them to achieve the device’s long-term environmental effect.
- Is it possible to manufacture in a way that saves energy and water?
- Is it possible to employ additive manufacturing to decrease material waste?
- Is my contract manufacturer adhering to environmental best practices for carbon capture and waste stream disposal?
- Can I improve the manufacturing process with my current resources?
- Are my employees satisfied with their well-beings, safety and working environment throughout the operation process?
- Packaging : Packaging has significant environmental impacts, which encourages most businesses to reduce their use of it.
- How can I reduce packaging waste?
- Is it possible to utilise recyclable or biodegradable packaging materials?
- Is it possible to remove the usage of plastic or petroleum-based foam packing elements?
- Can my customers cooperate with me to reduce or return or reuse the packaging waste?
- Can I move from paper-based documentation to on-device content when practical?
- Distribution : Distribution plays a key role in reducing carbon impact, but global markets make it challenging. Businesses can optimise by selecting production and storage sites that reduce long-distance transport and adopting technologies like electric vehicles.
- Can production and warehouse sites be selected to reduce long-distance transportation?
- How can I design the distribution schedule to maximise transportation efficiency?
- How can I work with my customers to improve the current distribution?
- End-of-life : High safety standards limit efforts to reduce disposable components. Concerns over hazardous waste, biological contamination, and the cost of sterilisation have hindered the shift from disposable to more sustainable devices.
- Is it possible to make the transition from a single-use device to a reusable device?
- Can the device or its components be recycled? Could it be returned to the manufacturer for reconditioning?
- If thrown away, what are the environmental impacts of the device? How can I minimise that?
- Do I need special waste treatment service for the device?
Medical device manufacturers must consider broader sustainability factors, including environmental impact (e.g., waste management, sustainable energy) and social responsibility (e.g., health and safety, diversity, community engagement).
In addition to safety, usability, reliability, and durability, sustainability is becoming a key focus to meet global market demands and ensure long-term growth. At Micro Systems, our expert teams collaborate with customers to integrate sustainability throughout the entire lifecycle of medical devices, from design to the application of innovative technologies, driving both progress and sustainability in the industry.
Check out our 2022 CSR Report here!
Contact us today at info@microsystems.uk.com to discuss with our experts at Micro Systems (UK).

Micro Systems specialises in the design, manufacture and validation of ultra precision micro moulds for the medical, pharmaceutical and optical markets, at the same time, the development and use of micro and nano technologies in the design and manufacture of injection moulded components. We have a dedicated micro moulding facility, and have ISO13485 and ISO9001 certifications. For more information, please Contact us or visit our website.