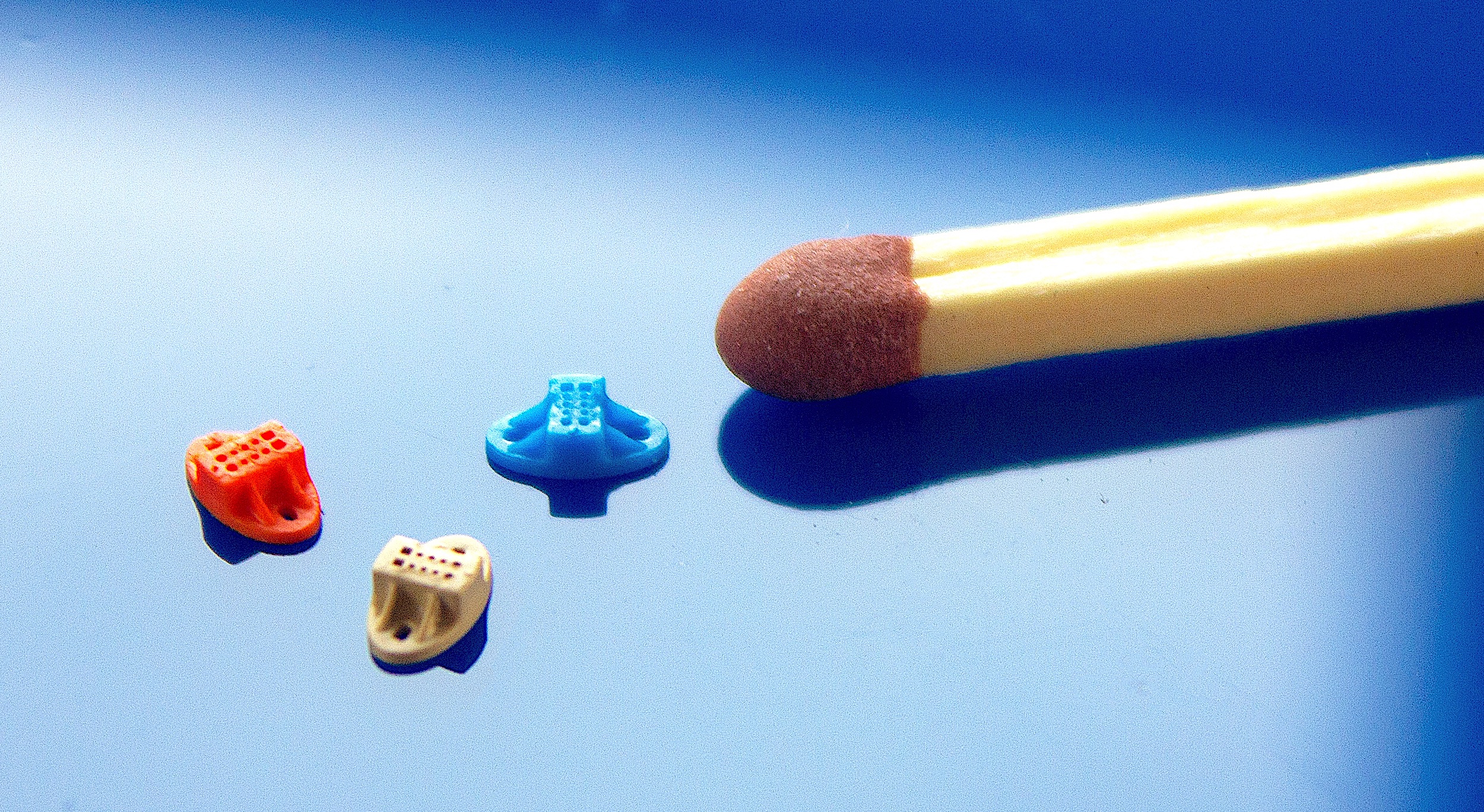
The commercialisation of Microfluidic devices

(Photo: Micro Systems)
What is Microfluidics?
Microfluidics could be defined as the science and engineering of systems in which the behaviour of fluid is different from conventional flow theory mainly due to the small length scale of the system (Nguyen et al., 2019). Microfluidics combines the profound knowledge within the field of the physics of fluids, at the same time, the engineering and expertise of designing and manufacturing devices with extremely small features.
The microfluidics market has steadily grown, reaching USD 20.14 billion in 2021 and projected to grow at a CAGR of 16.1%, reaching USD 77.28 billion by 2030, driven by rising healthcare demand. The market’s expansion is fuelled by innovations such as 3D-printed, paper-based, and droplet-based microfluidics, alongside the integration of AI and machine learning for real-time analysis and decision-making in diverse applications.
Mass manufacturing methods for microfluidic devices are crucial for transitioning from laboratory prototypes to commercialisation and clinical testing. The process involves several stages, including design, prototyping, optimisation, micro-tool manufacturing, precise replication, surface treatment, and device integration. Plastic is a key material in mass production due to its cost-effectiveness, flexibility, and adaptability, making it widely used in the industry.
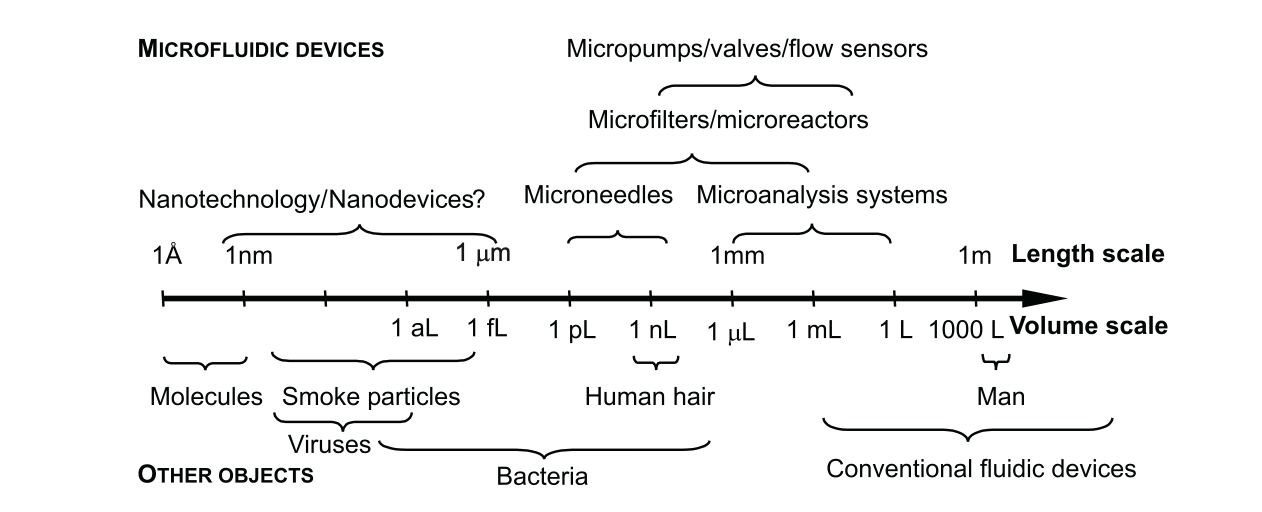
Microfluidic device sizes vary greatly depending on their applications (Photo: Nguyen et al., 2019)
Factors affecting the commercialisation of Microfluidic devices
Microfluidic applications offer the potential to reduce turnaround times and costs in analytical instruments, particularly in medicine, veterinary medicine, and environmental sciences. However, this breakthrough technology has only recently entered consumer goods. Over the past 20 years, several businesses have emerged to commercialise microfluidics, initially focused on biological studies and chemical synthesis to replace manual processing and large benchtop equipment. These businesses have embraced the lower costs of microfluidic devices, thanks to efficient reagent use, high-throughput capabilities, miniaturisation, and the ability to use inexpensive materials (Blow, Microfluidics: The Great Divide). However, slow commercialisation is due to factors like the need for additional training and external equipment (e.g. pumps, pneumatic fluid handling systems), limiting widespread application, especially in genomics and Point-of-Care (POC) diagnostics.
Many successful microfluidic companies have effectively combined product and customer development by focusing on market entry strategies. Despite its potential as a laboratory tool, microfluidics is still searching for its ideal applications, with few devices generating revenues above $100 million. As a result, many investors favour other cutting-edge technologies with established market niches (Blow, Microfluidics: In Search of a Killer Application). To overcome commercialisation challenges, greater focus is needed on the standardisation and integration of microfluidic devices.
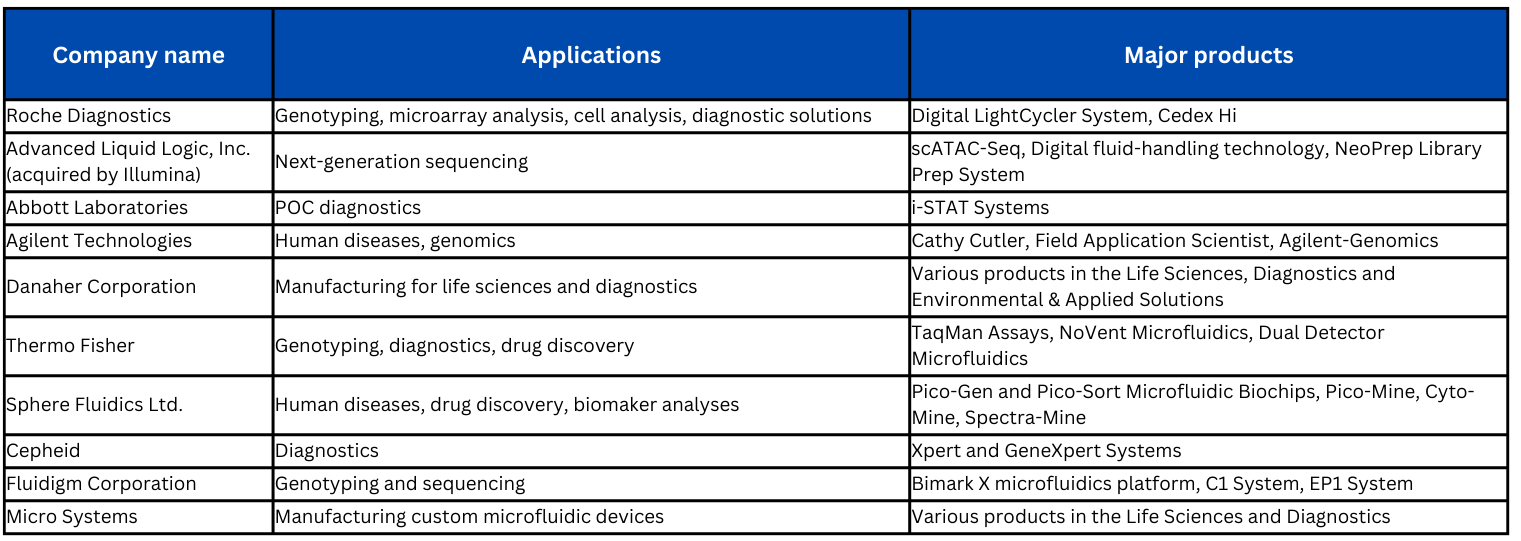
Some leading companies in the innovation and manufacturing of microfluidic devices
There has been a lack of collaboration between the development and integration of microfluidic components, with academic research often being unfocused. Successful commercialisation requires close collaboration between researchers and engineers from the design stage to ensure that the final product maximises the potential of LOC devices at an affordable cost. To drive microfluidics adoption, greater industry-academic cooperation is needed. Industry partners with marketing expertise should actively engage in discussions on market success potential, while academic researchers focus on developing fully integrated products for specific applications. Balancing academic publishing, business partnerships, and patent protection is essential for maximising societal impact.
In the past decade, hundreds of microfluidics articles have been published, with annual publications increasing (Mark et al., 2010). However, only a few practical devices have made a significant societal impact (Van den Berg et al., 2010). As users prioritise functional technology, academia must shift its focus from publishing advanced science to developing practical, commercially viable applications that deliver real-world benefits.
Another key factor is standardisation for microfluidic-based medical devices, which offer the guidelines for dimension and performance control between microfluidic connections, accurate flow rate monitoring, trustworthy testing of various parts or device characteristics, and a modular method to integrating microfluidic, electric, and optical functions (Reyes et al., 2021). So far, there have been some standards developed by ISO (International Organisation for Standardisation), greatly benefiting in creating a controlled and unified supply chain in business. From a production perspective, it eliminates the unnecessary labour and time in the device design process, particularly prototypes. Nevertheless, setting up a standardisation system is complicated, especially in the field of microfluidics when there are so many possible uses and manufacturing methods, hence more joint efforts between researchers, designers and engineers are required.
Other factors hindering the commercialisation of microfluidic devices include long development period (Dekker et al., 2018), high failure rate and complicated production method (Tian) and high cost of R&D and manufacturing (Nguyen et al., 2018).
For the commercialisation of microfluidic devices in the coming years, microfluidic researchers and engineers are aiming for inventions at a lower cost with a higher application level on a larger scale, based on the combined emerging technologies to address real-world issues.
References:
Nguyen, N.-T., Wereley, S.T. and Mousavi, S.S.A. (2019) Fundamentals and applications of Microfluidics. Boston: Artech House.
Blow, N. (no date) Microfluidics: The Great Divide, Nature News. Available at: https://www.nature.com/articles/nmeth0909-683 (Accessed: 30 June 2023).
Blow, N. (no date a) Microfluidics: In search of a killer application, Nature News. Available at: https://www.nature.com/articles/nmeth0807-665 (Accessed: 30 June 2023).
MARK, D., HAEBERLE, S., ROTH, G., VON STETTEN, F., & ZENGERLE, R. (2010). Microfluidic lab-on-a-chip platforms: requirements, characteristics and applications. Chem Soc Rev, 39(3), pp.1153-1182.
VAN DEN BERG, A., CRAIGHEAD, H. G., & YANG, P. (2010). From microfluidic applications to nanofluidic phenomena. Chemical Society Reviews, 39(3), pp.899-900.
Reyes D. R. et al., “Accelerating innovation and commercialization through standardization of microfluidic-based medical devices,” Lab Chip 21, 9–21 (2021). 10.1039/D0LC00963F
Dekker S. et al., “Standardized and modular microfluidic platform for fast lab on chip system development,” Sens. Actuators B 272, 468–478 (2018). 10.1016/j.snb.2018.04.005
Niculescu A. G., Chircov C., Bîrcă A. C., and Grumezescu A. M., “Fabrication and applications of microfluidic devices: A review,” Int. J. Mol. Sci. 22, 2011 (2021). 10.3390/ijms22042011
Nguyen H. T., Thach H., Roy E., Huynh K., and Perrault C. M. T., “Low-cost, accessible fabrication methods for microfluidics research in low-resource settings,” Micromachines 9, 461 (2018). 10.3390/mi9090461
Micro Systems’s vast know-how in design, ultra-precision micro machining capabilities and expert knowledge in micro moulding technology allow us to manufacture advanced microfluidic moulds with tolerance as low as +/-0.001mm, with integrated optics. We have a dedicated micro moulding facility, and have ISO13485 and ISO9001 certifications. For more information, please Contact us or visit our website.