What are ISO Cleanroom Classifications for Medical Devices?
Industries like life sciences, aerospace, optics, and nuclear require highly controlled, hygienic environments. Manufacturers use ISO Cleanroom classifications to meet strict product standards.

What is ISO Cleanroom?
According to ISO 14644, cleanrooms and controlled environments are specifically designed to manage contamination levels suitable for sensitive operations. A cleanroom is defined as a space engineered to control the introduction, generation, and retention of particles, as well as maintain specific concentrations of airborne particles to meet strict operational requirements.
What are ISO Cleanroom Classifications?
Within the ISO Cleanroom, there are 9 classifications, ranking from ISO 1 to ISO 9. The technique assigns a score based on the size and amount of airborne particles per m3, with 1 being the cleanest cleanroom environment. Simply put, the air in the room is cleaner the lower the number.
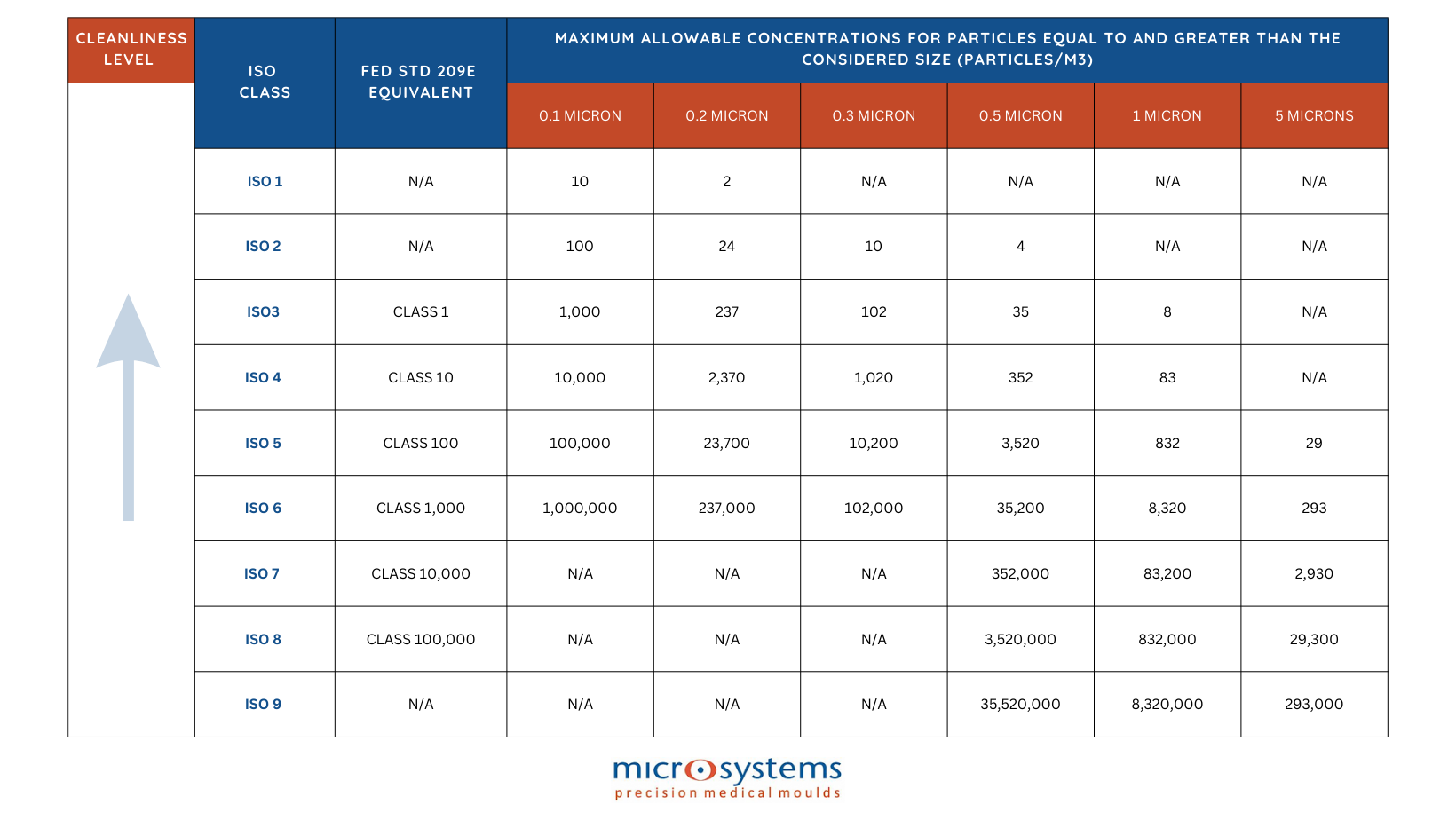
The particle size to be filtered is a critical factor in cleanroom design. ISO 1 cleanrooms, which require no more than 0.23 particles at 0.1 micron per square meter, are exceptionally rare, with only a few facilities globally capable of maintaining such stringent conditions.
For example, an ISO 7 cleanroom must limit particles smaller than 5 microns to fewer than 2,930 per cubic meter. To put this into perspective, a human hair is approximately 100 microns in diameter, and the human eye can only detect particles at least 50 microns in size—demonstrating the microscopic scale of a micron.
The cleanliness requirements of a cleanroom depend on the sensitivity of the work being performed. The more susceptible a product is to contamination, the higher the cleanliness standard required. To maintain these standards, the cleanroom must be meticulously controlled for cleanliness, temperature, humidity, pressure, air changes per hour, and flow rate.

What ISO Cleanroom Classifications for Medical Devices?
The manufacturing of medical devices typically occurs in cleanrooms with ISO classifications ranging from 5 (Class 100) to 8 (Class 100,000). Packaging processes for medical devices are commonly performed in ISO 7 (Class 10,000) or ISO 8 (Class 100,000) cleanrooms, often with an ISO 8 gowning room.
ISO 7 cleanrooms, compared to other classifications, are more cost-effective to construct and require less energy due to their lower air change requirements. These rooms necessitate an airlock at all entry points, maintain internal air pressure between +25 and +30 pascal, and require 30-60 air changes per hour. Suitable materials for ISO 7 cleanrooms include smooth, durable, and easy-to-clean options like hard walls, monobloc, PVC, and glass, all of which are resistant to common detergents.
Do’s and Don’ts inside Cleanroom
A comprehensive risk analysis is essential for cleanroom integrity, with mitigation strategies outlined in Standard Operating Procedures (SOPs). To maintain cleanliness standards, personnel must undergo regular SOP training, and access should be restricted to authorised individuals only.
Do’s:
- Leave all personal items outside of the cleanroom before entering the cleanroom.
- Wear proper cleanroom gowning materials and protection kit before entering the cleanroom.
- Practise self-control to avoid scratching, touching body parts, etc. Practise good personal hygiene.
- Clean all surfaces regularly with dedicated cleaning equipment to remove contamination.
- Always keep all doors closed to maintain the required pressure and environment cleanliness.
- Avoid running or moving quickly than necessary in the cleanroom.
Don’ts:
- Do not eat, drink or smoke in the cleanroom.
- Do not enter the cleanroom sick or feeling unwell.
- Do not wear heavy makeup, perfume, etc. inside the cleanroom.
- Do not turn off any fan filter units and other cleaning equipment.
Why choose medical device manufacturers with ISO Cleanrooms?
The healthcare sector’s strict regulations demand that medical device manufacturers comply with global standards to ensure product safety.
By working with manufacturers maintaining ISO Cleanrooms, OEMs can ensure compliance, secure certifications, and guarantee reliable production. Companies like Micro Systems offer operational control, efficiency, and adherence to sterility and global regulatory standards. At Micro Systems, a complete process from mould design through micro mould manufacturing and development to validation are carried out within an ISO Class 7 cleanroom, and backed up by an extensively equipped Metrology department to ensure the highest quality and assurance.

Micro Systems specialises in the design, manufacture and validation of ultra precision micro moulds for the medical, pharmaceutical and optical markets, at the same time, the development and use of micro and nano technologies in the design and manufacture of injection moulded components. We have a dedicated micro moulding facility, and have ISO13485 and ISO9001 certifications, and an ISO Class 7 Cleanroom. For more information, please Contact us or visit our website.